Despite intensified efforts to combat counterfeit medications, the Pharmacy and Poisons Board has revealed the presence of counterfeit batches falsely labeled as Truvada, a widely used HIV prevention drug worldwide. The board, via its official communication platform, has issued a stern warning of “severe legal and regulatory consequences” for individuals involved in trading, distributing, selling, or dispensing from these fraudulent batches. Authorities in Kenya are concerned that a significant number of counterfeit Truvada drugs may have already infiltrated the market.

According to UNAIDS data from 2022, approximately 1.4 million people in Kenya are living with HIV, with 1.2 million receiving antiretroviral therapy drugs. Truvada, manufactured by Gilead Sciences Inc. based in the United States, is utilized for the treatment of HIV as well as a preexposure prophylaxis for individuals at high risk, including those with multiple sexual partners and intravenous drug users who share needles.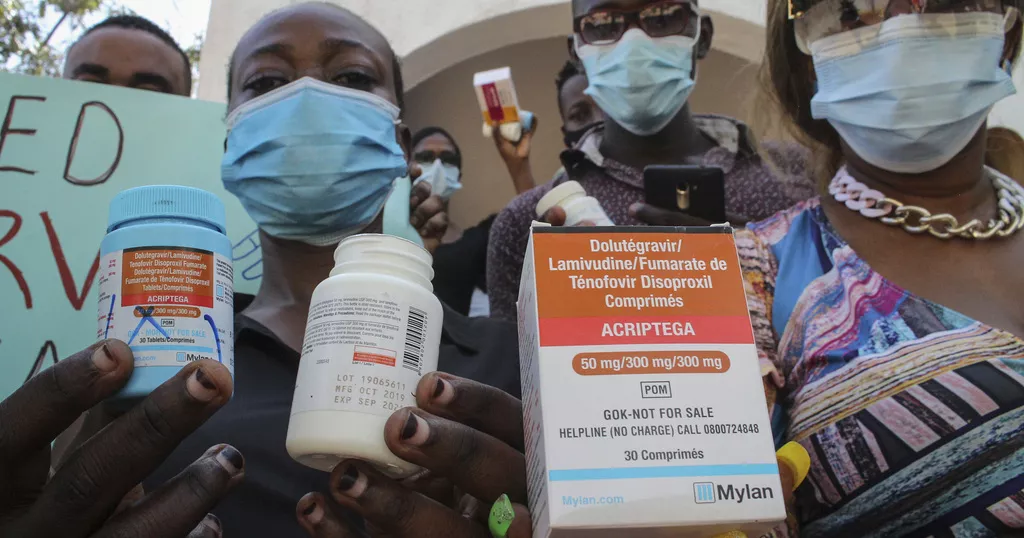
Earlier this month, the National Syndemic Diseases Control Council in Kenya, a government body responsible for coordinating the national strategy for HIV and AIDS, raised concerns about a 61% increase in HIV infection rates among individuals aged 15 to 29 between 2021 and 2022. This discovery of counterfeit Truvada drugs in Kenya, the commercial hub of East Africa, underscores the formidable challenge in combating the proliferation of fake medicines.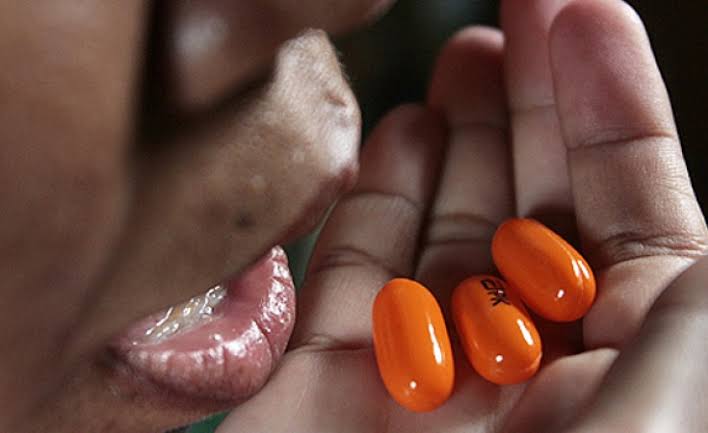
Health workers across Africa have expressed apprehension about complacency regarding AIDS treatment as improvements are made in accessing appropriate care.
